Vasileia Ismini Alexaki Group
Cell metabolic reprograming in metabolic and inflammatory disease

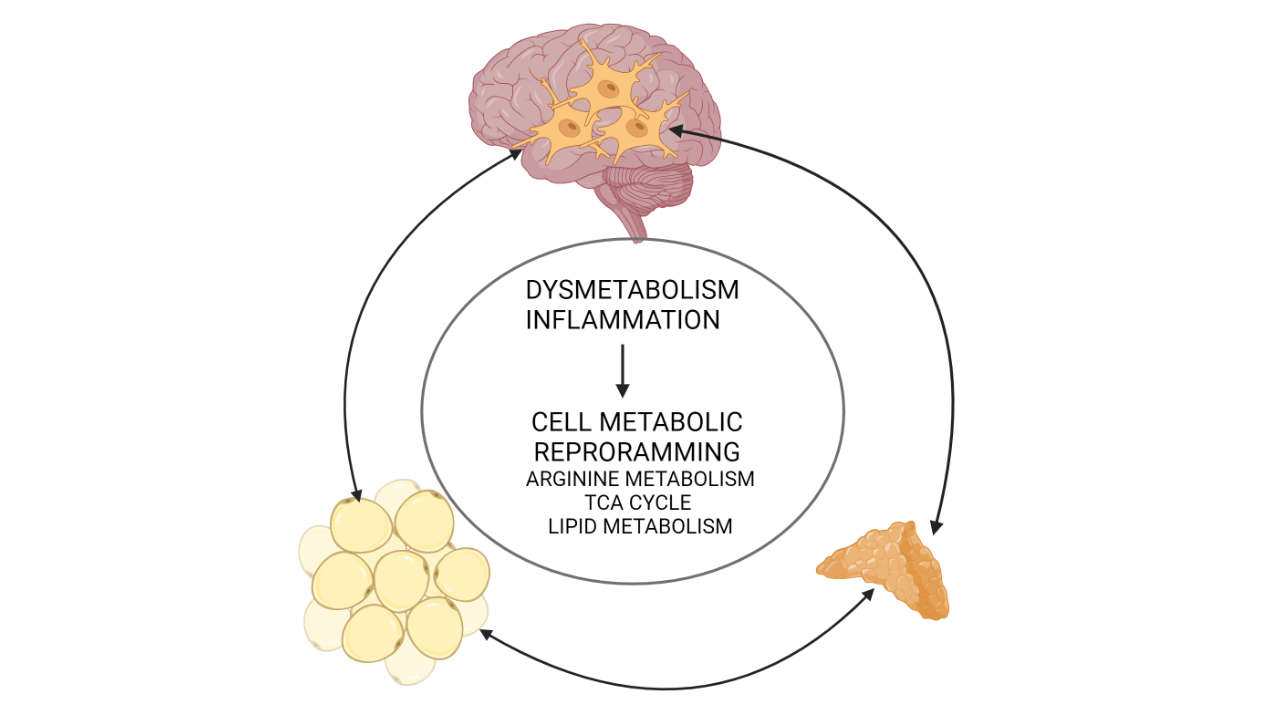
Our work explores the reprograming of cell metabolism in metabolic disease in different tissues. We aim to understand which metabolic adaptations are protective and which are maladaptive and identify key metabolic components that can be targeted to normalize tissue physiology. Diet-induced obesity is a very common metabolic disease with detrimental consequences such as cardiovascular disease, liver disease, cancer, chronic stress and depression. Although initially affecting the adipose tissue, which acts protectively by storing excess lipids through its enormous expandability, obesity gradually has systemic consequences. The current view is that this is due to inflammatory and metabolic spill-over from the hypertrophic adipose tissue to the circulation, which affects other organs, such as the liver, the vasculature or the brain. Our aim is to understand, which are the immunometabolic pathways involved in these processes. For instance, we recently found that polyamine metabolism is key in preserving adipose tissue health, as it inhibits hypertrophy, inflammation, senescense and fibrosis, while promoting macrophage-mediated resolution of inflammation. We also investigate whether polyamines may be involved in other anti-adipogenic mechanisms, such as regulation of glucocorticoid signaling in adipocytes. As obesity is linked to chronically increased circulating amounts of glucocorticoids, i.e. stress hormones promoting insulin resistance, central obesity, neurodegeneration and many other perturbations if chronically elevated, we also aim to understand the mechanisms underlying obesity-associated chronically increased glucocorticoid production. We recently showed that obesity is linked to lipidomic changes in adrenocortical cells leading to increased glucocorticoid production, thereby adding to the hypothalamus-hypophysis-adrenal axis a second level of regulation of glucocorticoid production at the adrenal cortex. Mechanistically, we identified fatty acid desaturase 2 (FADS2), the rate-limiting enzyme of polyunsaturated fatty acid (PUFA) synthesis, as a target that can be inhibited by dietary compounds to normalize glucocorticoid production in obesity.
However, we are also interested in revealing immunometabolic pathways regulating the responses of cells to inflammation per se. For instance, we recently showed that acute inflammation can impair adrenocortical function, involving perturbation of the TCA cycle at the levels of succinate dehydrogenase. In the past, we investigated how adrenocortical hormones affect peripheral and brain inflammation. Linking to the latter, we now explore the immunometabolic circuitry underlying acute and chronic microglia-mediated neuroinflammation. For instance, we found that TCA cycle reprograming occurs upon inflammatory activation of microglia providing negative feedback on inflammatory responses. We currently investigate whether this adaptation is protective or rather detrimental in demyelinating disease.
In sum, we aim to understand the adaptive and mal-adaptive changes in cell metabolism determining cell fate and function. As outlined above, in the context of metabolic disease we focus on the triangle adipose tissue, adrenal gland and brain trying to understand metabolic changes at the cellular level and inter-organ communication.
Future Projects and Goals
In the next years we will be exploring cell metabolic adaptations in different brain cell types (neurons and glia) in the context of metabolic disease (obesity), demyelinating/neurodegenerative disease, as well as aging (which is also associated with chronic inflammation and dysmetabolism). We will lay particular focus on microglia (main phagocytes and innate immune cells of the brain) and try to dissect the metabolic frame in which their adaptations are protective, and beyond of which they become detrimental or insufficient. Of note, up to now very little is known around metabolic adaptations in microglia. Dissecting the metabolic circuitry in brain cells is expected to set the basis for the development of a new drugs for the treatment of neurodegenerative disease.
Methodological and Technical Expertise
- diet-induced obesity, in vivo metabolic analyses, mouse models for (neuro)inflammatory disease
- FACS sorting
- primary cell isolation and culture (macrophages, microglia, adipocyte progenitors, adrenocortical cells etc)
- functional / metabolic analyses
- transcriptomic (scRNA-seq, ChiP-Seq, RNA-seq), proteomic, metabolomic profiling