Stephan Speier Group
Islet of Langerhans Physiology in the Pathogenesis and Therapy of Diabetes

© CRTD
Background
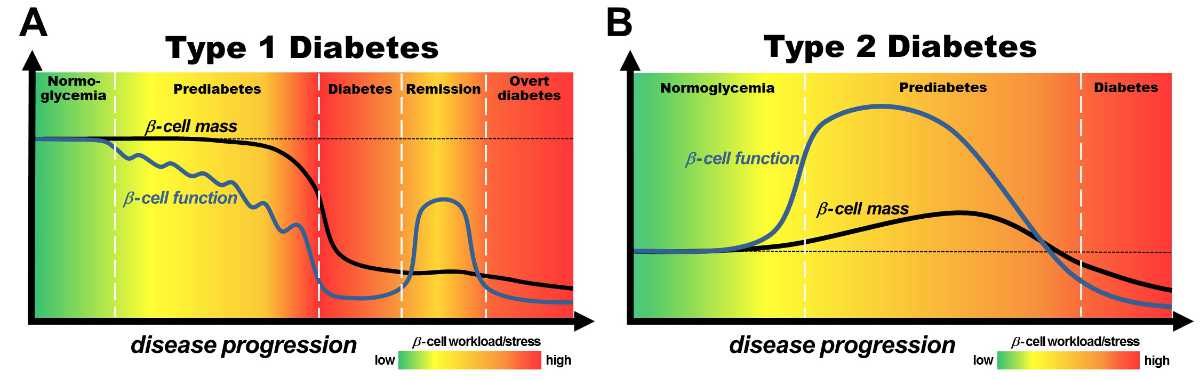
Diabetes is a growing epidemic with major impact on life style, health and life expectancy of the affected patients. Diabetes develops when systemic insulin concentrations are insufficient to control blood glucose homeostasis. This shortage in insulin is either caused by a functional deterioration of insulin secreting pancreatic beta cells or by a loss of their mass (reduced cell number). Recent research by our and other groups has shown that in different phases of type 1 and type 2 diabetes pathogenesis the contribution of changes in beta cell mass and/or function varies (→ Figure 1).
Based on these findings we believe that successful treatment of diabetes depends on targeting beta cell dysfunction and mass loss in specific phases of pathogenesis.
Thus, our group investigates the role of beta cell mass and function at distinct stages of diabetes pathogenesis and aims to discover novel therapeutic targets for beta cell protection and recovery in type 1 and type 2 diabetes. A central aspect of our research is the translation of our findings into the human setting by utilizing novel platforms to study human pancreatic tissue and islets. In addition, we utilize our unique platforms to optimize alternative cell sources for the successful generation of cell replacement therapies for diabetes.
Approach
The unique approach of our lab is the study of islet cell physiology within an intact tissue environment or the systemic setting. For that purpose we have established novel in situ and in vivo techniques to complement standard methods of islet research. Thereby, we aim to account for the numerous local and systemic signals which affect the physiology and pathogenesis of islets within the organ tissue and inside the living organism.
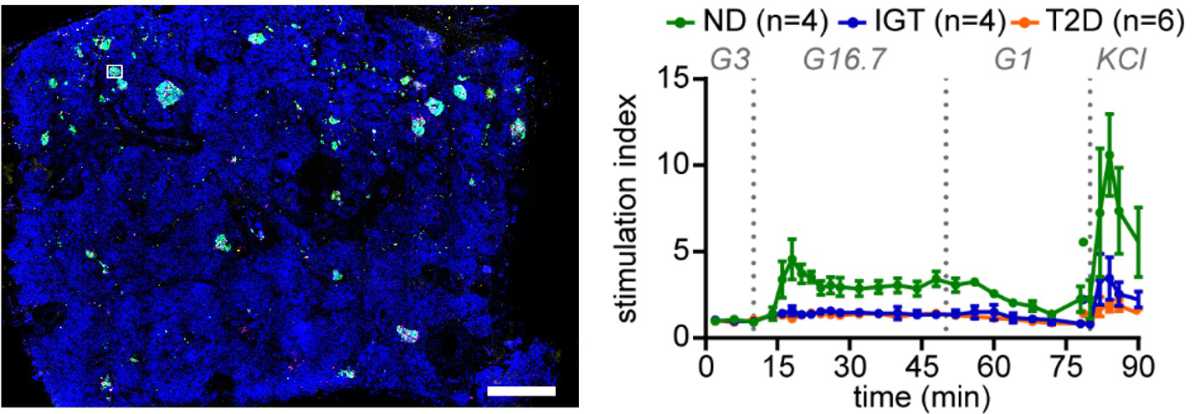
For example, the pancreas tissue slice platform allows us to study the human pancreas from organ and tissue donors to assess for the first time human islet cell pathophysiology within an intact organ tissue environment (→ Figure 2).
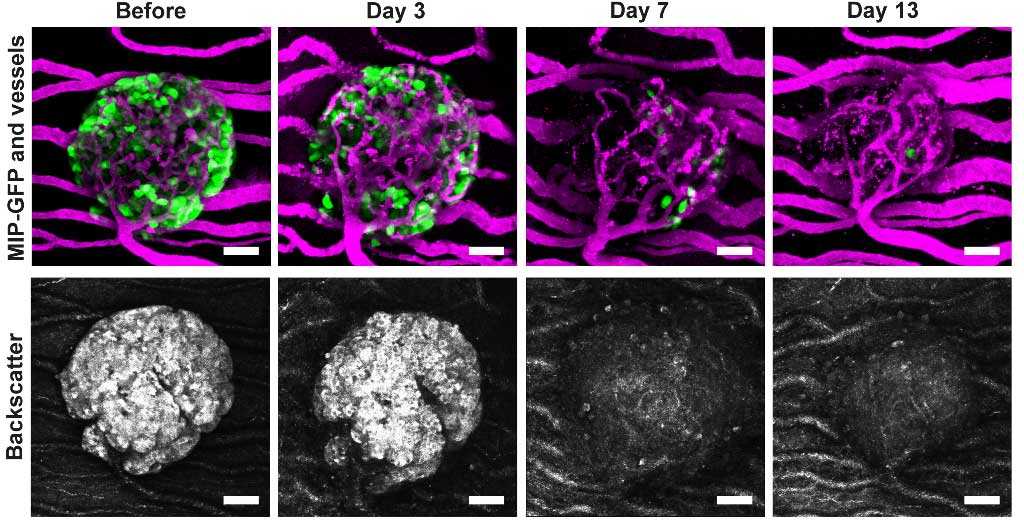
In addition, our in vivo imaging platform facilitates the noninvasive longitudinal observation of islet physiology in a systemic setting for prolonged time periods. This unique setup enables for the first time to study islet cells during changing metabolic conditions and disease pathogenesis (→ Figure 3).
Future Projects and Goals
- Assessing the mechanism of beta cell dysfunction in diabetes pathogenesis
- Facilitating the development of cell therapies for diabetes
- Translation of research findings to human
Methodological and Technical Expertise
- in vivo, in situ and in vitro two-photon and confocal laser scanning microscopy
- patch-clamp technique
- assessment of cell and systemic physiology in animal models
- novel platforms to study human islets of Langerhans and pancreas tissue