Natalia Rodríguez Muela Group
Selective neuronal vulnerability in neurodegenerative diseases

Why a given neuropathology does not impact all neurons of the same type to a similar level? This is a fascinating question in neurodegeneration research that remains unanswered and represents the main focus of my lab.
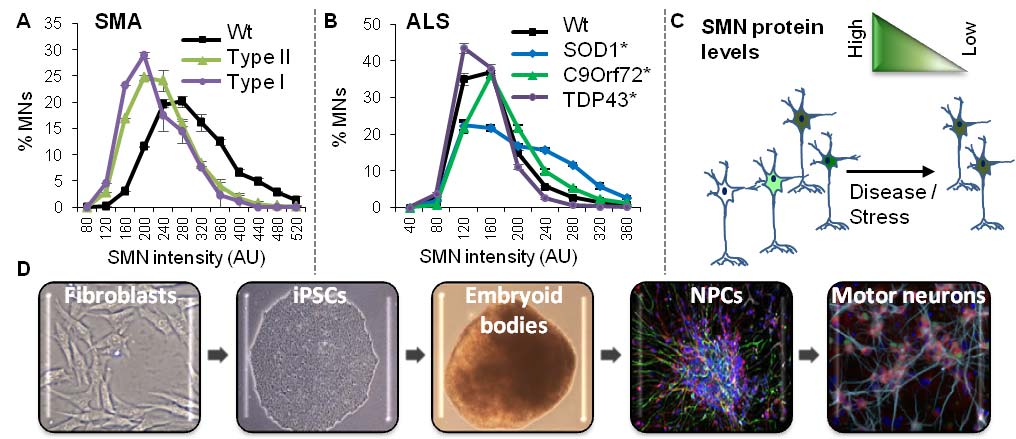
In most neurodegenerative diseases certain neuronal subgroups degenerate fast while others, carrying the same mutations – if any –, subjected to theoretically analogous stress and displaying comparable functional properties remain unaffected, even at the latest stages of the disease. Motor neuron diseases are a group of disorders in which motor neurons (MNs) of the spinal cord and/or the motor cortex are the primary cell type affected where this perplexing feature can also be observed. This key aspect of motor neuron diseases had been difficult to address using available mouse models. We use as main model system a human induced pluripotent stem cell (iPSC)-based approach and discovered that even within a single genetic background, MNs display a wide diversity of SMN levels – protein essential for MN survival –, and that those cells with low SMN are more susceptible to die. This correlation holds true in MNs expressing mutations causing the most common motor neuron diseases (amyotrophic lateral sclerosis or ALS and spinal muscular atrophy or SMA) and even in MNs obtained from unaffected patients. This discovery led to a new understanding of the importance of cell diversity in the progression of these diseases and helped to explain why there is a “survivor” population of MNs. However, there is so much that we still ignore:
- What are the main similarities and differences between the surviving ALS and SMA MNs?
- Are there factors/pathways shared between neuronal types unaffected in MNDs that explain their protection?
- What is the molecular basis of SMN heterogeneity across MN populations?
- Ultimately, why do many neurons degenerate while some remain fully resistant?
Using a variety of approaches ranging from cellular biology techniques and live imaging to genome editing and single cell genomics, and utilizing hiPSC together with transgenic animal models, we investigate the molecular mechanisms underlying such selective MN death. We devote a special focus at exploring the molecular basis of proteostasis failure – major causative event in neurodegeneration –, and at examining the contribution of cell autonomous and non-cell autonomous factors to the selective MN loss. Our ultimate goal is to understand the molecular origin of this distinctive neuronal vulnerability to design effective therapeutics targeting specific neuronal subtypes or phases of the disease.
Future Projects and Goals
- Investigate the origin of the heterogeneity in the levels of proteins essential for MN survival.
- Explore cell-autonomous similarities and differences between surviving and affected SMA and fALS MNs.
- Explore the contribution of a dysregulation of the autophagy-lysosome axis to the selective MN loss.
- Unravel the molecular mechanisms that explain the protection of other neuronal types unaffected in motor neuron diseases.
Methodological and Technical Expertise
- Mouse and human pluripotent stem cell culture and neuronal differentiation
- Autophagy activity analysis
- Cellular/molecular biology techniques
- Fluorescence microscopy and time-lapse imaging
- CRISPR/Cas-mediated genome editing