Michele Solimena Group
Cell Biology of Pancreatic Beta Cells and Pathogenesis of Diabetes

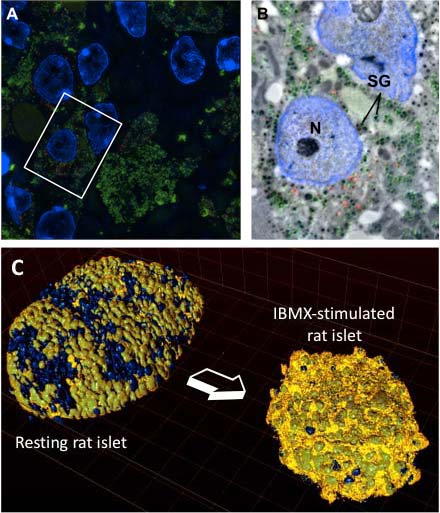
Our laboratory is interested in the cell biology of pancreatic beta cells, which produce and secrete insulin. A deficient output of insulin secretion relative to metabolic demands is the ultimate cause of all forms of diabetes mellitus. Beta cells store the insulin hormone within organelles termed secretory granules, which undergo fusion with the plasma membrane and release insulin in response to high levels of circulating glucose. Prolonged glucose stimulation depletes beta cells of insulin secretory granules, which must be quickly replenished.
The main question we are addressing is how beta cells regulate the turnover of insulin secretory granules, including their biogenesis, exocytosis and destruction. A mechanistic description of these processes may provide insight into the pathogenesis of type 1 and type 2 diabetes and contribute to the development of novel approaches for its treatment. These studies may also have general implications in the field of neuropeptide and peptide-hormone secretion from neurons and other endocrine cells.
Specifically, we are addressing the following key questions:
- How is a beta cell counting its own insulin secretory granules?
- Which are the molecular signatures associated with granule aging?
- What accounts for the reduced mobility and propensity to undergo exocytosis of older versus younger insulin secretory granules?
- Which posttranscriptional mechanisms allow glucose and other stimuli to prompt the rapid biogenesis of insulin secretory granules?
- Which retrograde signaling pathways couple the exocytosis of insulin secretory granules with regulation of beta cell gene expression and replication?
Moreover, we are pursuing:
- siRNA and small compound high-throughput screenings for the identification of genes and drugs of potential interest for the therapy of diabetes
- the identification of genes differentially expressed in pancreatic beta cells of euglycemic and type 2 diabetic subjects as well as of individuals with impaired glucose tolerance secondary to insulin resistance
- the elucidation how enteroviruses potentially implicated in the pathogenesis of type 1a diabetes may trigger autoimmunity against insulin granule proteins
Future Projects and Goals
To address these questions we employ a large array of quantitative imaging, molecular, and biochemical approaches using insulinoma cultured cells, isolated pancreatic islets and genetically modified mice as main model systems. We actively collaborate with structural biologists, biophysicists, bioinformaticians and mathematicians to deepen our understanding at the level of single molecules and to elaborate in-silico predictive models of these processes. Through the collaboration with clinicians at our University we extend our investigations to human islets from non-diabetic as well as diabetic subjects.
Methodological and Technical Expertise
- RNA and protein biochemistry
- TIRF microscopy
- CLEM (Correlation light electron microscopy)
- Transgenic mice
- Laser capture microdissection