James Sáenz Group
Synthetic Membrane Biology

© B CUBE
Our laboratory is focused on understanding how lipids contribute to membrane function and organismal fitness from synthetic protocells to microorganisms. We are interested in topics ranging from how membranes contributed to the origin of cellular life, to identifying membrane-based targets for antibiotic resistance. One of our central aims is to mine a repertoire of biological innovations from the simplest microorganisms to elucidate the minimal set of principles and components required to assemble a robust and autonomous synthetic cell membrane.
Cells are complex interconnected systems with many processes occurring in parallel The complexity and robustness of the cell is crucially dependent on the ability to orchestrate localization of a staggering number of simultaneously occurring biochemical processes. Lipid membranes play a central role in this partitioning of bioactivity. Membranes serve a dual role as a selectively permeable barrier, and as a two-dimensional solvent hosting biochemical activity (e.g. signaling, transport, growth and division). The ability of membranes to perform these crucial roles rests on the collective interactions of lipids and proteins, and the physical and chemical membrane properties resulting from these interactions. Our lab broadly aims to understand how lipids modulate cell membrane function and to apply this knowledge to engineer synthetic biological systems from the bottom up.
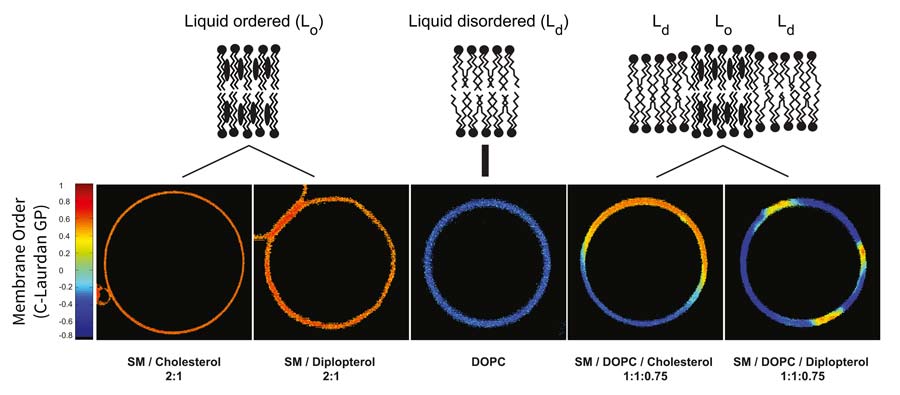
I did my postdoctoral work at the MPI-CBG with Kai Simons investigating what preceded sterols in the evolution of membrane organization and elucidating the molecular basis for such organization in bacterial membranes. I demonstrated that hopanoids, ancient bacterial lipids, are biophysically similar to sterols in their ability to order membrane lipids and to form liquid ordered domains (Saenz et al., 2012). This observation alters our understanding of membrane evolution, suggesting that the emergence of ordered biochemically active liquid membranes, and thus the ability to subcompartmentalize membranes, preceded the evolution of sterols. This not only suggests that liquid-ordered membranes may be common outside the Domain Eukarya, but it decouples the evolution of this trait from the requirement for molecular oxygen. Following on these discoveries, I began work to understand the biological role of sterol analogues in bacterial membranes. I discovered that hopanoids interact with glycolipids in bacterial outer membranes to form a highly ordered bilayer in a manner analogous to the interaction of sterols with sphingolipids in eukaryotic plasma membranes (Saenz et al., 2015). This revealed a convergence in the architecture of bacterial and eukaryotic membranes and implicated the biosynthetic pathways of hopanoids and other order-modulating lipids as potential targets to fight pathogenic multidrug resistance.
Future Projects and Goals
Moving to the B Cube in Fall 2016 my group will begin work on the design principles of functional synthetic membranes from the bottom up. This work will be motivated by the question “Why did life choose lipid membranes?” and “What are the minimal features of a membrane that are necessary to support life?” We will pursue the following aims:
- Understand how lipid ordering affects membrane organization and protein dynamics and function using bacterial model organisms. In particular we will elucidate the role of bacterial sterol analogues in tuning membrane protein function and dynamics, and the link between lipid ordering and mechanisms of antibiotic resistance.
- Elucidate the minimal set of features of a membrane that are required to support life. This work will begin by trying to elucidate the simplest means to construct a responsive synthetic membrane capable of adjusting its composition to maintain robustness in response to environmental changes (e.g. temperature and pH). The design of such a minimal membrane will be inspired from the architecture of Mycoplasma, some of the simplest free living organisms.
- Explore how RNA-lipid interactions could have contributed to the emergence of cellular life and how these interactions can be applied to design novel responsive synthetic membrane systems.
Methodological and Technical Expertise
- Lipid Chemistry
- Lipidomics
- Microbiology: cultivation, genetics, physiological characterization
- Membrane biophysics
- In vitro model membrane systems