Franziska Knopf Group
Biology of bone regeneration

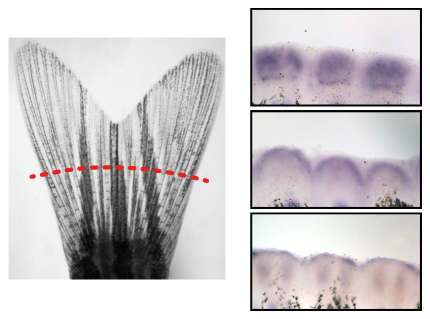
Although bone is a regenerative tissue also in humans, it can be permanently lost in circumstances of trauma, cancer or degenerative diseases. Due to their high regenerative capacity zebrafish have emerged as a powerful tool to study bone regeneration. In particular, bone forming and bone degrading cells (osteoblasts and osteoclasts) can be visualized easily in zebrafish, thus allowing their observation in the living organism. This can be used to monitor osteoblast and osteoclast function throughout processes of bone formation and bone degradation, and to study cross-talk of bone cells with other types of cells, such as immune and cancer cells, in the bone niche.
In the lab we make use of pharmacologic approaches and molecular biology tools to investigate adverse effects of certain drugs used in the clinics. We have analyzed the anti-regenerative effects of immunosuppressive glucocorticoids in zebrafish, in which bone forming osteoblasts are fluorescently labelled. In doing so, we found that both osteoblast activity and proliferation are reduced by glucocorticoid exposure. In addition to this, we are interested in uncovering the molecular impact of glucocorticoids on innate immune cells, which contribute to bone regeneration. To preserve bone strength, we aim for the identification of anti-inflammatory molecules counteracting bone destruction.
A main focus of the lab is to understand underlying mechanisms of successful bone regeneration. Zebrafish regenerate bone quickly after injury, both in appendages and the skull; however, they do not overgrow bone. How do they do so? To address this question, we explore ways of positively and negatively influencing tissue regeneration. This is feasible both by drug treatments and genetic modulation. By using a combination of these approaches with state-of-the-art imaging technologies, we hope to identify mechanisms that are involved in growth control of bone and of regenerating tissues in general. Our in vivo imaging approaches are also employed to study the early metastatic events leading to tumor cell engraftment in bone tissue. Our work will have important implications for future regenerative therapies of the skeleton and help to identify targets to fight bone metastasis.
Future Projects and Goals
We will exploit the regenerative capabilities and excellent imaging capabilities of zebrafish to
- investigate growth and patterning underlying successful bone regeneration,
- promote zebrafish as a preclinical model to characterize adverse effects of widely used anti-inflammatory steroids, and to
- better understand metastasis of cancer cells in bone.
Methodological and Technical Expertise
- Bone injury models
- Intravital microscopy in zebrafish
- Genetic and pharmacological modulation of gene expression