Ellen Adams Group
Physical Chemistry of Biomolecular Condensates

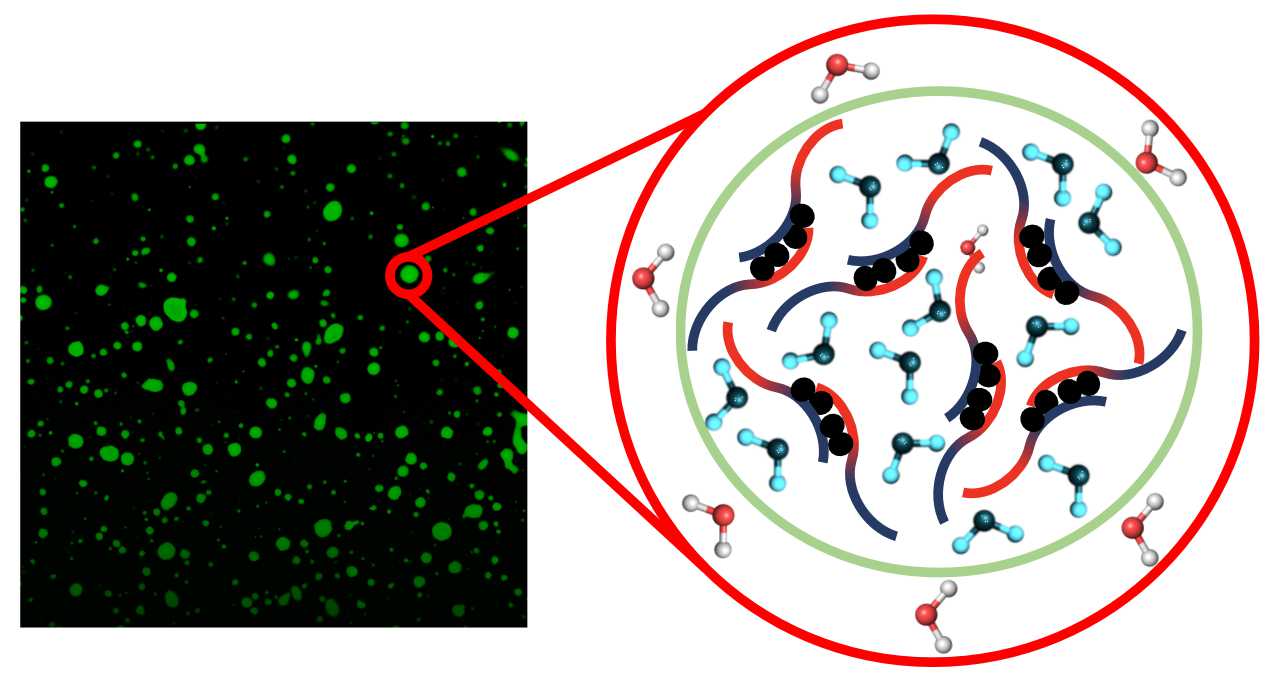
Liquid-liquid phase separation (LLPS) occurs in living cells, in which liquid-like membrane-less compartments form. These protein enriched biomolecular condensates regulate biochemical and biophysical processes, but have been linked to formation of protein aggregates found in neurodegenerative diseases. LLPS stems from intrinsically disordered proteins (IDPs), where protein-protein interactions between regions of low complexity have been shown to be a major driving force. However, little is known about the role of the water solvent in this process. Why do two coexisting liquid phases that are entropically unfavorable form? How do properties of water inside biomolecular condensates differ from water outside? How do protein-water interactions, i.e. hydration water, contribute to driving LLPS?
Our group studies macroscopic and microscopic solvent properties of biomolecular condensate systems. We use linear and nonlinear spectroscopic methods to probe the structure and dynamics of bulk and interfacial water. We aim to understand the underlying thermodynamic properties of protein-water interactions that lead to LLPS.
Future Projects and Goals
- Hydration properties of biomolecular condensates
Proteins have extended dynamic hydration shells that are fundamental to their structure and function. Using THz spectroscopy, we probe the structural properties of protein hydration water and determine how this drives biomolecular condensate formation. - Solvent dynamics of protein systems
Formation and aging of biomolecular condensates are dynamical processes in which material properties are altered. We use pump-probe spectroscopy to determine how solvent dynamics are correlated to macroscopic changes within protein systems. - Interfacial properties of biomolecular condensates
Biomolecular condensates are membrane-less organelles, but little is known about the molecules that comprise this interface. With the use of the surface selective sum frequency generation spectroscopy, we aim to unravel a molecular picture of these interfaces.
Methodological and Technical Expertise
- THz-ATR spectroscopy
- THz time domain spectroscopy
- Pump-probe spectroscopy
- Sum Frequency Generation Spectroscopy