Andreas Linkermann Group
Cell Death

Regulated Necrosis
Necrosis is a regulated process. It is genetically determined and mediated by clearly identified signallaing pathways. Upon plasma membrane rupture, cells release their intracellular content, (so-called damage associated molecular patterns (DAMPs)).
Pathways of Regulated Necrosis include but are not limited to necroptosis and ferroptosis. We demonstrated that necroptosis and ferroptosis are independent signaling pathways that contribute to the overall picture organ damage. We therefore believe that targeting both pathways with combinatory therapies may be the most effective treatment.
Necroptosis
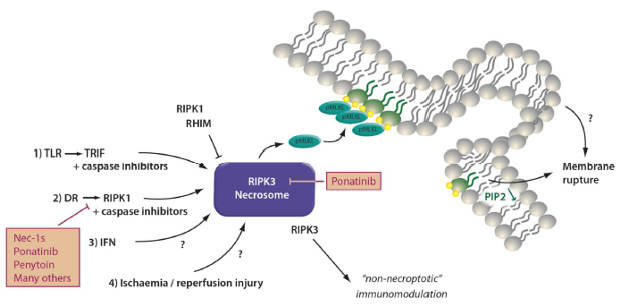
The signaling pathway of necroptosis: Phosphorylated mixed linage kinase domain like (pMLKL) is the only known effector of necroptosis. Receptor interacting protein kinase 3 (RIPK3) phosphorylates MLKL at defined phosphorylation sites in its “activation loop”. Downstream of pMLKL, sensitivity of cells to undergo necroptosis is controlled by proteins that control membrane blebbing and macrovesicle formation.
Several triggers result in the formation of the necrosome. Death receptor (DR), e.g. TNFR1-stimulation in the presence of a caspase-inhibitor or a dysfunctional caspase-8 represents the most prominent and best investigated stimulus that requires RIPK1 kinase activity for necrosome formation. RIPK1, as RIPK3, contains a rip homotypic interacting motif (RHIM)-domain that intercalates with the RHIM of RIPK3 and prevents necrosome assembly. Inhibitors of RIPK1 kinase activity, such as Nec-1s and ponatinib, maintain the inactive state of RIPK3 potentially by keeping RHIM-RHIM-interactions intact. Necrosome assembly has been repeatedly reported downstream of Toll like receptors that bind to the intracellular adapter protein TRIF which also contains a RHIM domain and activation of this pathway results in robust RIPK1-independent necrosome formation. In vivo, reperfusion following ischemic injury severely triggers necroptosis, and several other models have been described, such as injection of recombinant human TNFα into mice. DAI/ZBP1 is a protein that senses viral DNA and also activates the necrosome machinery. Necroptosis contributes to acute kidney injury (AKI) in some models, such as ischemia, but inhibition of necroptosis does not affect other models, such as foliac acid induced AKI.
Ferroptosis
Peroxidation of membrane phospholipids represents the point of no return during ferroptosis that results in loss of NADPH-abundance and synchronized regulated necrosis (SRN) in sensitive organs, such as the renal tubular compartment or the myocardium. Lipid peroxidation is typically inhibited by glutathione peroxidase 4 (GPX4), a selenoenzyme that requires glutathione (GSH) to function. Ferroptosis may be triggered by inhibition of system Xc-minus, a cys/glu-antiporter in the plasma membrane by a lethal compound referred to as erastin. Inhibition of system Xc-minus functionally inhibits the activation of the GSH-synthase (GSSG), resulting in GSH depletion, dysfunction of GPX4 and ferroptosis. Apart from GPX4, the FSP1 oxidoreductase system prevents ferroptosis in a GSH-independent manner.
Ferroptosis accounts for the majority of tubular cell loss during AKI because of its mechanism of SRN that damages a complete functional unit. Ferroptosis is a great target for future AKI therapies.
Future Projects and Goals
- Identification of the relative contribution of necroptosis, ferroptosis and pyroptosis to overall organ damage (e.g. during ischemia-reperfusion injury)
- Development of inhibitors/activators of clinically relevant cell death
- Identification of the most prominent necroinflammatory pathway for autoimmunity
- Studying the role of inflammation following ferroptosis
- Assessment of DAMPs as drivers of necroinflammation
Methodological and Technical Expertise
- cell culture molecular cell death research
- in vivo models of acute kidney injury (ischemia-reperfusion injury, cisplatin-toxicity, etc)
- in vivo models of shock and sepsis (TNF-shock, LPS-shock, clp-model etc)
- ex vivo models of renal tubule isolation from murine and human tissue
- medicinal chemistry – development of specific anti-necrotic/pro-necrotic small molecules