Ali El-Armouche Group
Novel therapeutic approaches for the prevention of lethal arrhythmias and heart failure progression

© Stephan Wiegand
The main focus of my group is to identify molecular mechanisms in arrhythmogenesis and heart failure progression with emphasis on novel nodal points in cardiac signaling. We aim to enhance the understanding of cardiac molecular signaling and to identify and promote new candidate targets for pharmacologic interventions. Our strategy is to use screening techniques to uncover new players followed by validation in gene targeted mice (conventional and conditional transgenesis) in order to test novel treatment strategies in a joint translational research activity.
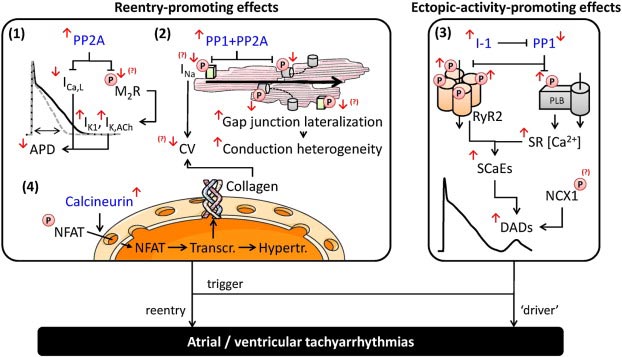
We are presently working on testing the therapeutic potential of protein phosphatase modulation (see figure, e. g. the PP1 inhibitor I-1) in arrhythmogenesis. By screening of human and experimentally failing hearts we identified I-1 to be markedly deactivated. Genetic ablation of I-1 protected the heart against lethal arrhythmias. In contrast, conditional overexpression (Tet-Off-system) of I-1 increased contractile performance, but also the propensity towards lethal arrhythmias. We therefore propose that pharmacological inhibition of I-1 may represent an attractive new therapeutic strategy.
As for I-1, we have initiated similar approaches for cyclic nucleotide phosphodiesterase (PDE) isoforms. Among the PDE superfamily, the PDE2 isoform is unique in being activated by cGMP. PDE2 is upregulated in human as well as in experimental heart failure but its physiological and pathological role in the heart remained unknown. We showed for the first that the physiological role of PDE2 is predominantly confined to heart rate regulation. Specific inhibition of PDE2 in dogs and mice led to an exclusive increase in heart rate, while heart-specific overexpression resulted in its decrease. Under chronic pathological conditions, PDE2 protected the heart from excessive sympathetic stress. Moreover, under acute stress, higher PDE2 abundance effectively protected against ventricular arrhythmia by reducing intracellular calcium leakage. Therefore we will start a gene-therapeutic approach by testing the benefit of AAV9-mediated PDE2 overexpression test how these interventions are compared to conventional beta-blockade (e.g. metoprolol as a mainstay in human heart failure therapy) in the setting of preexisting experimental heart failure in mice.
Future Projects and Goals
Heart failure has a higher prevalence and worse prognosis than most cancers. Stopping or reverting heart failure progression is a global challenge that requires new therapeutic paradigms. Our ultimate goal is to advance heart failure therapy and to prevent sudden cardiac death. To archive this aim we will elucidate on the mechanistical level and target for pharmacological intervention:
- Molecular mechanisms of heart failure and arrhythmogenesis with focus on signal transduction
- Cross talk mechanisms of myocardial phosphatases, phosphodiesterases and kinases
- Myocardial plasticity: hypertrophy, atrophy und remodeling
- Regulatory epigenetic mechanisms and histone modifying enzymes in the heart
- Redox-mediated signaling mechanisms in cardiac cells
Methodological and Technical Expertise
- Experimental heart failure and lifestyle mouse models
- Echocardiography in small animals
- ECG telemetry and electrophysiology
- Mouse genetics: Cre-LoxP system, knock-in technology, conditional technologies
- Small molecule high throughput screening
- Translation towards DNA-based therapies via AAV technology