Alf Honigmann Group
Membrane organization of cells and tissues

© TUD CMCB, M. Gonciarz
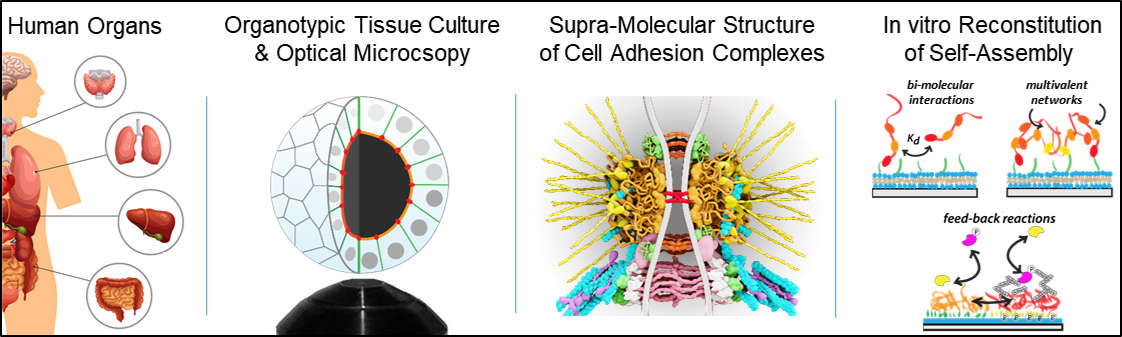
We aim to understand how tissue architecture emerges from the structure and dynamics of cellular interfaces. My group develops a combination of super-resolution microscopy, organotypic tissue culture, genetics and biochemistry to understand the molecular underpinning of how cells control the physical properties of their cell membranes (lipid composition, adhesion, cortical tension and osmotic gradients) to shape tissues.
Our recent work on epithelial tight junctions revealed a new mechanism how adhesion complexes self-assemble via cell-cell contact mediated phase separation of scaffolding proteins. In addition, we have reconstructed the supra-molecular architecture of the apical junctional complex in 3D epithelial tissue, which provides an important molecular/structural resource to understand how epithelial cells couple cell-cell interfaces to the cytoskeleton, apical polarity and transcription. An exciting discovery is that we can control 3D tissue shapes by manipulating the molecular structure of tight junctions. We are now using this feature in a VW funded project to study and engineer tissue folding mechanisms in 3D tissue culture systems.
Overall, our approach to making scientific discoveries often involves the concept of reconstituting emergent functions from the interactions of its underlying components. The beauty of reconstitution experiments is that the overwhelming complexity of biological systems can be picked apart and broken down to essential mechanisms. We use this approach to understand how molecular interactions drive formation of supra-molecular complexes like adhesion junctions as well as how cellular interactions lead to organ specific tissue architectures.
Future Projects and Goals
- Role of membrane remodelling in polarization and lumen formation in organoids
- Understanding how cells measure and control physical properties of cell their interfaces during morphogenesis (organoids).
- Visualizing lipid trafficking and metabolism in cells and tissues with high resolution microscopy
Methodological and Technical Expertise
- 3D organoid culture and genetic engineering
- Microscopy: STED, SPIM, FLIM, FCS…
- Laser ablation, photo-conversion, opto-genetics
- Protein purification and reconstitution with membranes
- Image analysis