Alexander Gerbaulet Group
Hematopoietic stem cell homeostasis and regulation

The Gerbaulet group studies the biology of hematopoietic stem and progenitor cells in their native environment. Our goal is to understand how blood formation proceeds in mammals, which factors govern and control this and how dysregulation of hematopoiesis leads to disease.
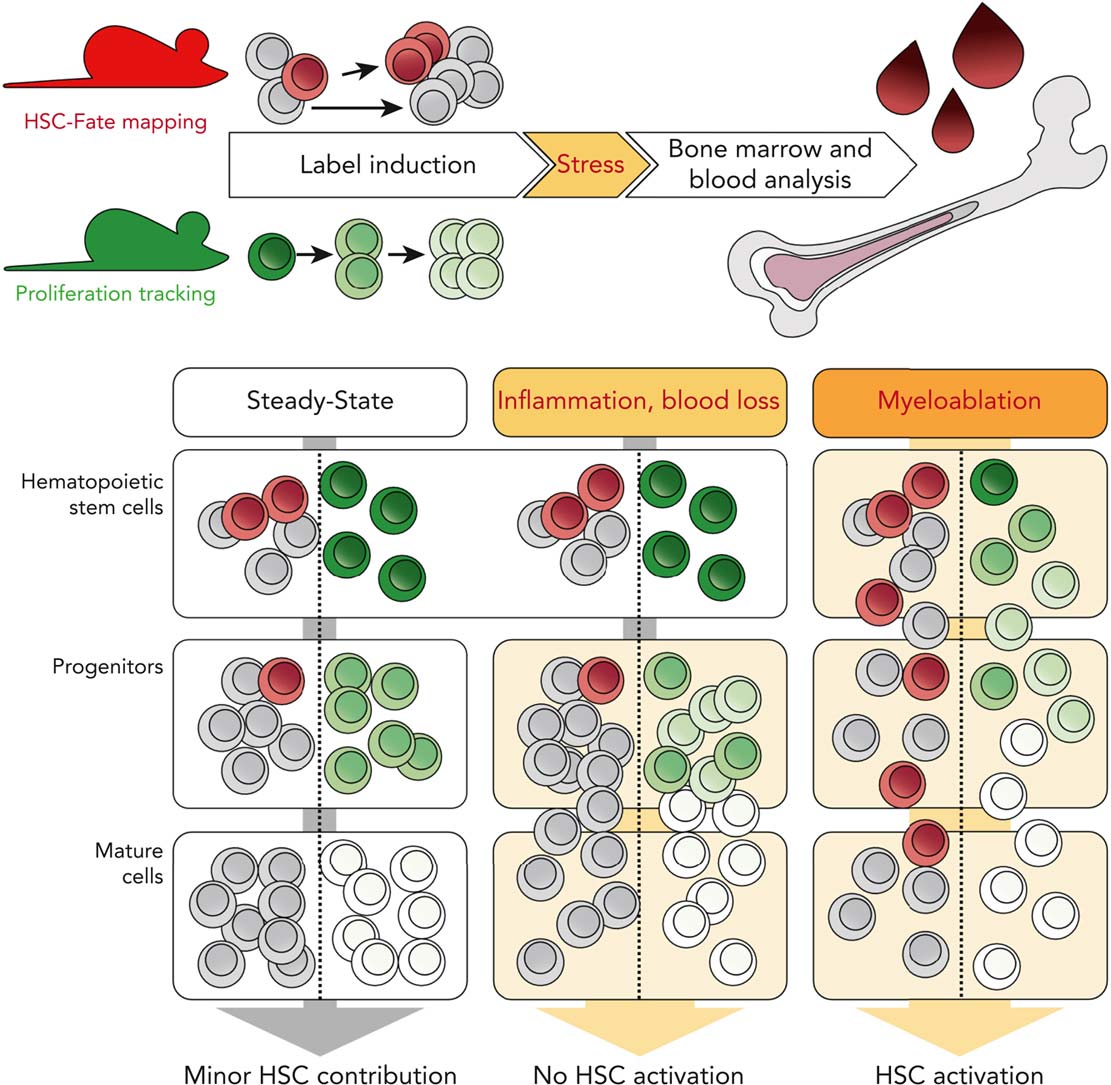
The hematopoietic system is the most regenerative mammalian tissue. A human being produces approximately 3 × 1011 mature blood cells per day, representing 90% of all cells regenerated on a daily basis. Hematopoietic stem cells (HSCs) are at the top of the hematopoietic hierarchy and can differentiate into all types of mature blood cells throughout life. HSCs were first identified by transplantation, in which recipient mice were preconditioned with ionizing radiation and reconstituted with donor bone marrow. This experimental paradigm revealed the existence of a rare population of somatic stem cells that can stably reconstitute all blood lineages lifelong. For decades, hematopoietic stem and progenitor cells have been studied primarily through such transplantation-based assays. However, recent work has shown that post-transplant hematopoiesis differs significantly from native hematopoiesis. Preconditioning of recipients by lethal irradiation or chemotherapy forces transplanted hematopoietic stem and progenitor cells to realize their maximum potential, but this does not necessarily reflect their fate and behavior in the native bone marrow environment.
To investigate the proliferation and differentiation of hematopoietic stem and progenitor cells in their physiologic environment, we use state-of-the-art mouse models. For the analysis and modeling of our experimental data, we collaborate with theoretical scientists to achieve a more quantitative interpretation of native haematopoiesis. We recently unraveled an alternative pathway of thrombopoiesis, in which HSCs directly differentiate into megakaryocytes, bypassing the classical multipotent progenitor intermediates (Morcos, Li, Munz et al., Nature Communications 2022).
HSCs are thought to be a reserve population residing in a deeply quiescent ("dormant") state that protects them from metabolic and proliferative stress. According to this concept, HSCs are activated by an increased demand for mature blood cells in situations such as systemic infection or severe blood loss. Using our fate mapping and proliferation reporter mouse models, we recently investigated how native hematopoiesis responds to systemic inflammation or blood loss (Munz et al., Blood 2023). Contrary to current beliefs, we found that these challenges do not robustly stimulate HSC differentiation and proliferation. Only severe disruptive "myeloablative" conditioning, including high-dose ionizing radiation and chemotherapy, requires HSC input.
We also have a long-standing interest in mast cells and the rare human disease mastocytosis, which is characterized by an abnormal accumulation of these cells in various organs. Most patients with mastocytosis have activating somatic mutations in the receptor tyrosine kinase kit (CD117). Similar to tissue-resident macrophages, mast cells are of dual ontogeny and are generated during both fetal and adult ("definitive") hematopoiesis. In an ongoing project, we are investigating how this mast cell ontogeny modifies the effect of a somatic kit mutation and may explain the heterogeneous phenotype of mastocytosis.
Future Projects and Goals
- We are investigating native hematopoiesis following mutations that either reduce genome replication fidelity or predispose to leukemia development.
- We want to elucidate how hematopoietic perturbations and stressors are resolved on a clonal level by employing genetic barcoding.
- We want to understand how the hierarchical organization of mature blood cell regeneration from stem and progenitor cells shapes the accumulation of somatic mutations and hence the risk of developing blood cancer. We hypothesize that the continuous but rare differentiation of quiescent HSCs into rapidly dividing progenitors limits mutation accumulation in the hematopoietic system.
Methodological and Technical Expertise
- Animal models for fate mapping and proliferation tracking
- Cellular barcoding
- Multicolor flow cytometry
- Hematopoietic stem and progenitor cell transplantation