Simon Alberti Group
Organization of cytoplasm across space and time

© Katrin Boes
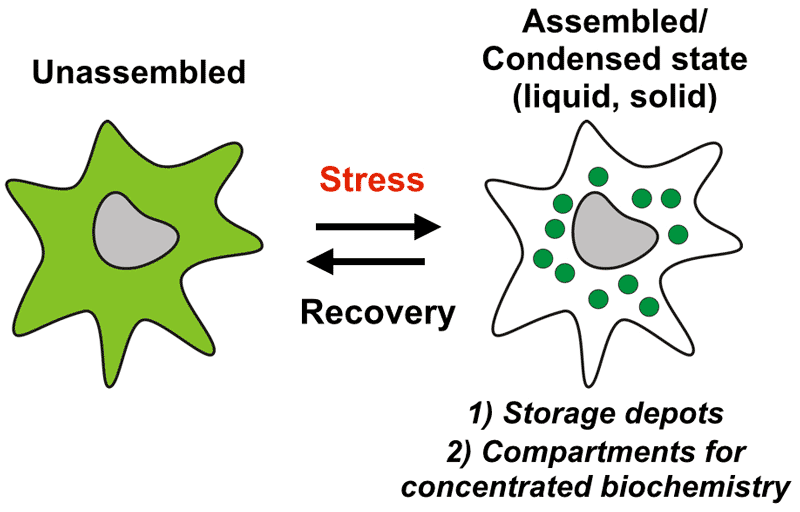
Many key biochemical reactions take place in the cytoplasm. We still know very little about the organization of the cytoplasm and the role of biomolecular condensates such as RNP granules in regulating cellular functions. My research group aims to elucidate molecular principles underlying the spatiotemporal organization of the cytoplasm by condensate formation. We are particularly interested in understanding how the cytoplasm changes upon environmental perturbations and stress (Figure 1). Stressed cells undergo controlled changes on many levels to alter their physiology and metabolism. Many of these changes result directly from alterations in the structure and organization of the cytoplasm. Understanding these changes and how they promote organismal survival is our key aim.
To investigate this question, we use cell biological, biochemical, biophysical and genetic approaches and diverse model systems, such as yeast, Dictyostelium, and cultured mammalian cells, allowing us to cover a wide range of organismal life styles. Our findings so far indicate that the mechanisms by which macromolecules form condensates are diverse and involve dedicated factors such as prion-like proteins and scaffold molecules such as RNAs.
Importantly, the ability to form such condensates comes with a cost, as many condensate-forming proteins have a high propensity to misfold and aggregate. Indeed, we could recently show that condensate-forming proteins have very unusual molecular properties and are associated with age-related diseases (Patel et al., 2015; Maharana et al., 2018). Thus, our long-term aim is to gain insight into the important link between the condensate-forming abilities of proteins and age-related protein misfolding diseases such as amyotrophic lateral sclerosis.
Future Projects and Goals
- Identify protein factors, domains, and sequence motifs that are required for the formation of condensates
- Identify molecular mechanisms regulating the formation of condensates, with a focus on the protein quality control machinery and post-translational modifications
- Genetic and chemical screens to identify modifiers of condensate formation
- Investigate how the ability to form condensates affects the physiological state of a cell, determines developmental decisions, and contributes to diseases and aging
Methodological and Technical Expertise
- Genetics and cell biology of S. cerevisiae, Dictyostelium, and cultured mammalian cells
- Fluorescence microscopy and time-lapse imaging
- Biophysical approaches such FRAP and photoconversion to study intracellular dynamics
- Biochemical reconstitution assays and in vitro and in vivo aggregation assays