Hayder Amin Group
Biohybrid Neuroelectronics (BIONICS) Lab

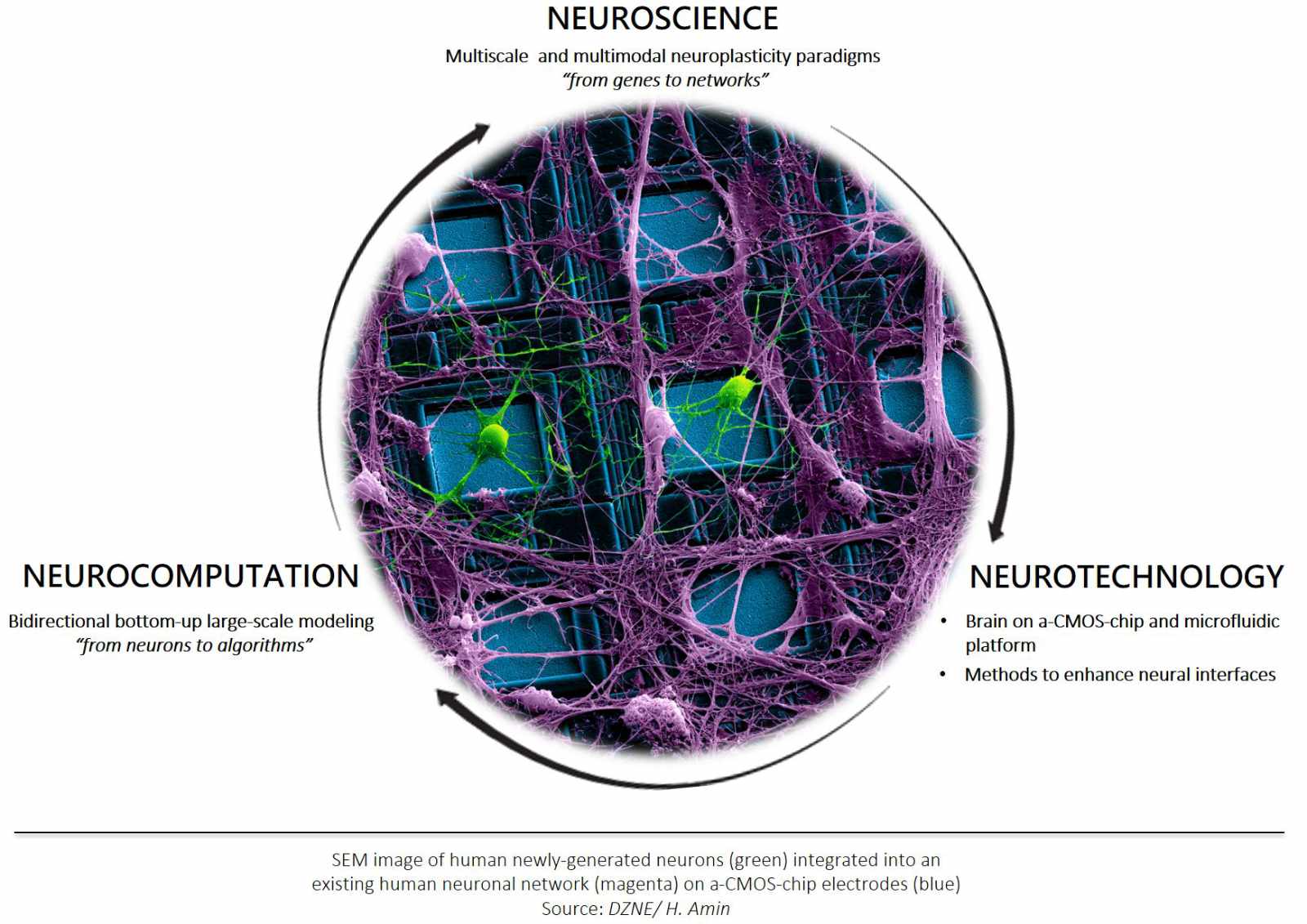
“We engineer a novel multiscale plasticity neuroelectronic (Plastronic) platform using a synergistic combination of techniques (see Figure) to decipher the molecular and functional plasticity simultaneously in a large-scale circuit/network in health and disease.”
Our lab is focused on understanding the interplay between molecular and cellular processes and functional plasticity consequences in neurophysiological states, aging, and neurodegenerative disorders. We use multidisciplinary experimental and computational approaches to examine multiple forms of plasticity as changes in the electrical and chemical signals in large-scale networks at single-neuronal details.
The adult brain is a uniquely plastic organ capable of generating new neurons throughout life to modify its structure and function to continuously learn and adapt to changes in everyday experience and environmental demands. These activity-dependent plasticity changes can be “functional”, where neurons adjust their electrophysiological properties. They can also be “molecular,” involving the regulation of particular gene expression patterns that are essential for the formation, maturation, and regulation of synaptic strength in those same neurons.
- But how are functional and molecular processes of plasticity interrelated?
- What is the functional role of newly-generated neurons in the large-scale adult brain?
- What is the mechanism underlying the integration of these neurons into a resident network?
- How do neural networks/circuits self-regulate to balance for plastic and stable neural code for performing a specific neuronal function?
- And importantly, how can the brain exploit new information of those new neurons to stall the consequence demands of aging and neurodegeneration?
To address these questions, linking electrophysiological, morphological, and transcriptomic data at a single neuron in a large-scale network is essential but has lagged due to the lack of multimodal and multiscale techniques. Our laboratory aims to circumvent these challenges by using advanced electrophysiological, imaging, molecular and cellular, and computation methodologies.
Diseases related to our field of research
- Alzheimer’s disease (AD)
- Amyloid-beta toxicity (Aβ)
- Parkinson’s disease (PD)
- Amyotrophic lateral sclerosis (ALS)
Future Projects and Goals
Our research themes are developed under the plasticity neuroelectronic platform and involve projects in the following areas:
- Neurogenic-plasticity models for aging and neurodegeneration: from genes to networks
We aim to characterize the activity-dependent adjustments (from genes to firing networks) of newly-generated neurons for the recruitment and functional integration into diseased or aged neuronal ensembles. - Excitatory/Inhibitory (E/I) balanced networks for empowering neuronal coding and synaptic stability
We study the long-term plasticity of excitatory and inhibitory (E/I) synaptic drive. We investigate how a balanced E/I regime confers robust, and high-dimensional neural codes and computational performance. - Engineering the brain-on-a-chip (microfluidic channels integrated with CMOS-MEAs)
We design and implement systemic microfluidic platforms on CMOS-MEAs to allow modeling different brain cell types for fundamental research, disease modeling, and drug development. - Bottom-up advanced computational analyses and modeling
Our integrative approach aims to create a bottom-up comprehensive network modeling to simulate multi-level experimental plasticity readouts (molecular, cellular, and circuits/networks). - Biofunctionalized platform for neural interfaces
We develop biofunctionalization methods to grow neuronal networks in well-defined configuration to investigate cell-to-cell and cell-extracellular matrix interactions. These methods also aim to improve the neuron-electrode interface to enhance the quality of the recorded neural signals.
Methodological and Technical Expertise
We build sophisticated approaches to index various neuroscience challenges from genes to networks by using:
- High-resolution CMOS-MEA (i.e., 4096 microelectrodes)
- Calcium imaging
- Patch-clamp recordings
- Genome-wide transcriptome profiling
- Opto-chemogenetic methods
- In-vitro hiPSC-derived neuronal cultures and ex-vivo transgenic mouse brain, olfactory bulb slices
- Molecular and cellular techniques
- Micro-/nanotechnology
- Computational modeling