Bernd Schröder Group
Proteolytic control of membrane trafficking and signal transduction

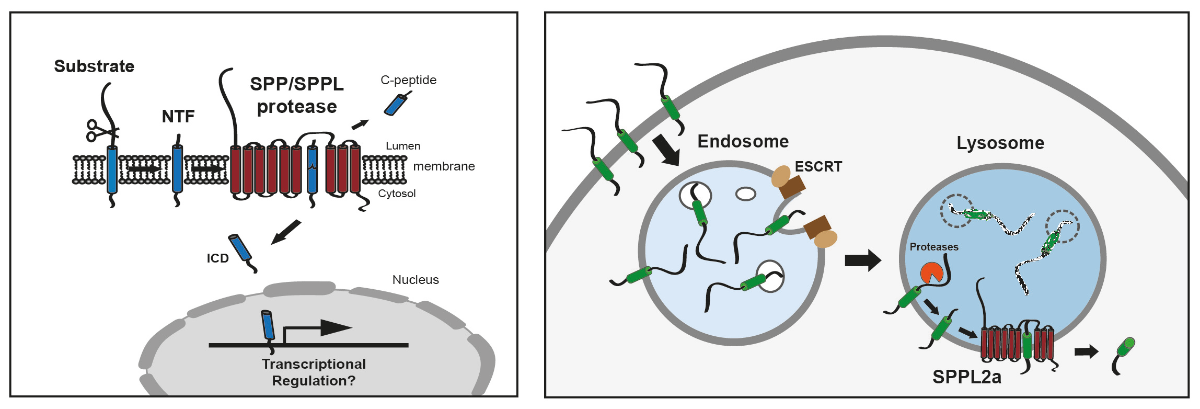
Regulated intramembrane proteolysis (RIP) has emerged over the last decades as an important principle of cellular signal transduction. This involves the proteolytic cleavage of substrate proteins within their transmembrane segment in the hydrophobic environment of the phospholipid bilayer which is often preceded by a proteolytic release of their ectodomain (shedding). As exemplified by the paradigmatic Notch and SREBP pathways, intramembrane proteases can release membrane-bound transcription factors. In addition to that, intramembrane proteolysis is a homeostatic process which initiates the degradation of substrate proteins and thereby controls their levels within cellular membranes. At the moment, we are working primarily on the SPP/SPPL family of aspartyl intramembrane proteases comprising the ER protein signal-peptide-peptidase (SPP) and the four homologous SPP-like proteases SPPL2a, SPPL2b, SPPL2c and SPPL3, which are localised in lysosomes, at the plasma membrane in the ER or the Golgi, respectively. SPP/SPPL proteases exhibit a similar catalytic centre like the presenilins, the catalytic subunits of the γ-secretase complex implicated in Alzheimer disease. Substrate spectra of SPP/SPPL proteases include single-span transmembrane proteins with a type II topology and in the case of SPP also tail-anchored proteins which has not been reported for the other family members yet.
First of all, we are interested in this fascinating process from a mechanistic perspective in order to reveal how it is conducted and regulated. However, we especially aim to understand the functional relevance of the individual cleavage events. In addition to the processing of selected signal peptides, SPP has been implicated in the ERAD process, of which the importance in mammalian cells is only partly understood. We currently characterise several novel substrates of SPP/SPPL proteases which include active receptor proteins as well as proteins with a direct role in membrane trafficking and/or fusion. Based on this, we analyse at the cellular level how the action of SPP/SPPL proteases modulates and controls cellular signal transduction and membrane trafficking pathways. Beyond that, we aim to translate these findings into an in vivo context by using various knockout and transgenic mouse models. By this means, we have been able to demonstrate a role of SPP/SPPL proteases in important (patho)-physiological processes including adaptive immunity, atherosclerosis, spermatogenesis, pathogen recognition/innate immunity and metabolic regulation. Our previous work has identified important functions of SPPL proteases especially in immune cells, in particular of SPPL2a which cleaves the invariant chain of the MHCII complex in B lymphocytes and dendritic cells and is critical for the homeostasis of these cell types in mice. Therefore, our current work has a certain, though not exclusive, focus on immune cells. The distinct phenotype of SPPL2a-deficient mice characterised by a differentiation defect of B lymphocytes and dendritic cells has highlighted this protease as promising drug target for immunosuppression. We aim to further explore the potential as well as the risks of a therapeutic SPPL2a inhibition. In humans, genetic SPPL2a deficiency was recently shown to induce a susceptibility to mycobacterial infection, of which we try to understand the underlying mechanism using our mouse models. These aspects are directly related to CD74 as a substrate of SPPL2a and the apparent capability of this protein to influence signalling and membrane trafficking in immune cells, which is still poorly understood at the molecular level and which we therefore try to characterise further.
Future Projects and Goals
In general, it is not well understood how SPP/SPPL intramembrane proteases and those of other families are interconnected with cellular proteostasis networks controlling the homeostasis of membrane proteins like the ESCRT or the ERAD pathways and possibly also autophagy which is something we would like to explore. This also relates to the mechanisms of substrate selection. Why certain proteins like CD74 depend on intramembrane proteases for their turnover while others utilise alternative pathways is entirely unclear. Similarly, deeper insights how the activity of intramembrane proteases is regulated are deeply required. We are confident that the extensive characterisation of novel substrates which we currently pursue will provide a basis to approach these questions.
Methodological and Technical Expertise
- Cell culture: standard cell lines as well as isolation and culture of primary murine immune cells
- Molecular biology: cloning techniques, transcript analysis by qRT-PCR, etc.
- Flow cytometry and other immunological methods including functional assays
- Protein biochemistry methods including protease assays and signalling pathway analysis
- Microscopy: confocal laser-scanning microscopy